Early Clinical Formulation
In today’s pharmaceutical landscape, poorly soluble and/or permeable compounds are common challenges. Our team excels in developing formulations that address these issues, enhancing bioavailability and stability for your candidate compound. With our combined expertise in solid form and formulation, we aim to significantly de-risk your molecule, paving the way for successful clinical drug product formulation.
Our objective is to craft a stable, practical formulation that effectively tackles your molecule’s challenges. To guarantee the robustness of our formulations, we conduct thorough in-vitro testing, including lipolysis, dissolution, and flux assays. Additionally, we provide comprehensive support for the technical transfer of the formulation to your chosen Contract Development and Manufacturing Organisation (CDMO).
Enhancing Bioavailability with Oral Formulation Approaches
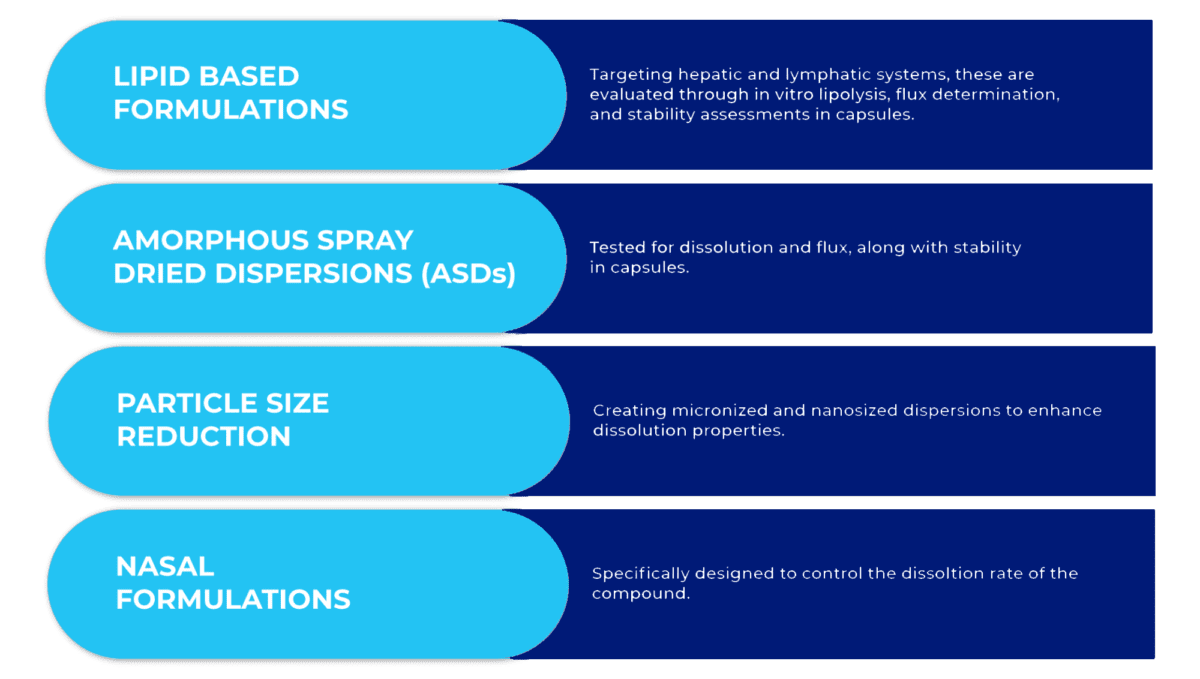
To address solubility, permeability, and bioavailability challenges in compounds, we employ several effective oral formulation strategies:
Common injectable formulation approaches used for improving the solubility, deliverable dose and stability of the compound include:
- Lyophilised buffered solutions for injection dosing. We pride in helping clients reduce the overall lyophilisation cycle times reducing costs and expediting the timeframe to clinic
- Solutions for injection where high drug loading is required
- Liposomal and emulsion systems which can add valuable IP to your program
All formulation work is supported by stability studies using Memmert chambers in typical ICH like conditions (25°C/60%RH and 40°C/75%RH).
