Bioavailability – can we improve it?
Bioavailability: What is it and what factors influence it?
Bioavailability (F) is a critical parameter in the developability of potential new drugs for unmet clinical needs. Where the bioavailability is a result of the structure of the molecule, there are technologies that might improve the performance, without changing the chemical structure.
Can we improve the bioavailability?
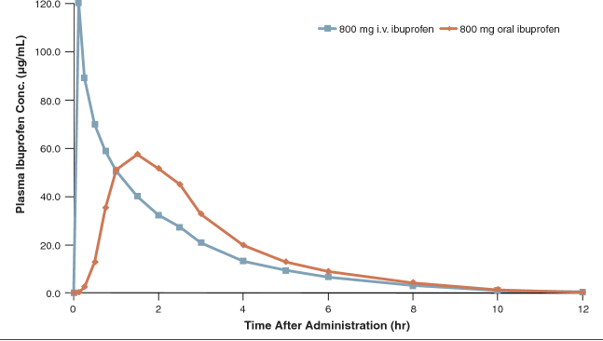 It’s a question we often get asked here at Sygnature Discovery. In our integrated projects, we assess bioavailability within the design–make–test cycle during the late lead optimization stage. When chemical alteration isn’t viable, our Form and Formulation Department’s comprehensive toolbox is utilized to potentially enhance bioavailability.
It’s a question we often get asked here at Sygnature Discovery. In our integrated projects, we assess bioavailability within the design–make–test cycle during the late lead optimization stage. When chemical alteration isn’t viable, our Form and Formulation Department’s comprehensive toolbox is utilized to potentially enhance bioavailability.
Customers who do not have an integrated project with Sygnature can still access Form and Formulation expertise through a fee-for-service model. Typically engaged during late lead optimization or candidate nomination stages, the dialogue often begins after initial PK experiments, transitioning into efficacy studies. At this juncture, customers seek insights into the dose necessary to elicit an in vivo model effect, considering that only the free drug concentration is effective. Recognizing that a drug’s bioavailability represents the fraction of orally administered drugs reaching systemic circulation, comprehending the interplay between dose, free concentration, and efficacy is pivotal for determining dosing strategies for subsequent toxicology studies and first-in-human trials.
The bioavailability of a compound is affected by both chemical and biological factors, which include:
- GI metabolism/stability
- Transporter influence
- Liver clearance (metabolism and biliary)
- Solubility/Dissolution
- Permeability
Collaborating with an integrated service partner can significantly conserve time, resources, materials, and finances. Any solution offered needs to address the ultimate question: Why is the bioavailability low? Initially, verification from the DMPK team is essential to rule out any inherent drawbacks in the compound. This involves confirming that the clearance rate is not excessively high, leading to consistently low free concentrations unable to induce an effective response. Once it has been established that bioavailability is not a function of liver clearance, the next step is to determine where the compound sits in the Developability Classification System (DCS).

The integration of formulation, DMPK, and Pharmacology into delivery at Sygnature Discovery.
The Developability Classification System
The DCS system is used to rank compounds in terms of ease of development. Solubility and permeability are the two key parameters that limit bioavailability. Compounds in DCS Class I, marked by high permeability and solubility, are the most straightforward to develop, while those in Class IV, characterized by low solubility and permeability, pose the greatest challenges. For compounds placed in Classes IIa-IV, formulation strategies can be employed to enhance their bioavailability.
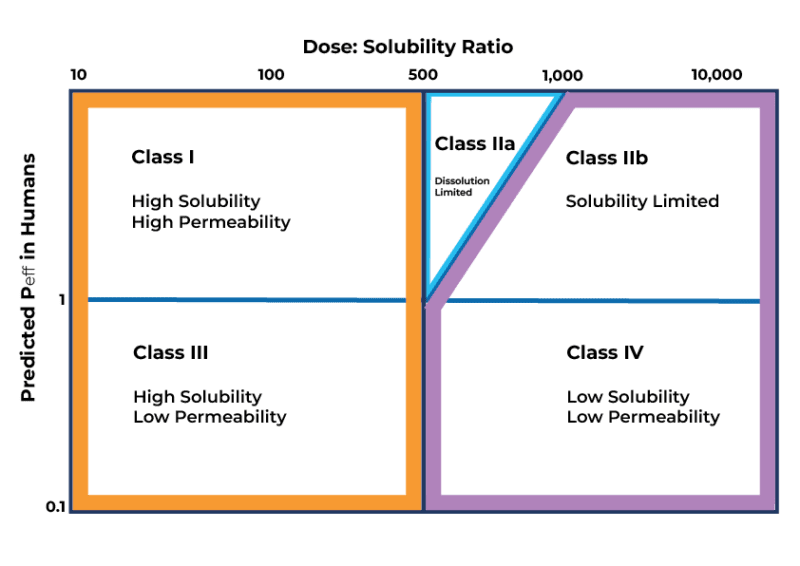
Formulation Strategies for Class IIa
Dissolution limitation affects Class IIa compounds. Enhancing dissolution primarily involves reducing particle size, thereby increasing the particle’s surface area-to-volume ratio and improving its dissolution capacity. Techniques such as micronization and nano-milling achieve particle size reduction.
Formulation Strategies for Class IIb
In contrast to Class IIa compounds, Class IIb compounds exhibit genuinely low solubility. Many of these compounds are crystalline. Modifying the solubility of a Class IIb compound involves altering its form, often through the creation of a new salt form or by developing a lyophilized or spray-dried amorphous solid dispersion.
Formulation Strategies for Class III
For Class III compounds, solubility is not an issue, but permeability is. Permeation enhancers and/or inhibitors of efflux substrates can be used to help maximise exposure.
Formulation Strategies for Class IV
Class IV compounds are the most challenging. When a client chooses to pursue the development of a Class IV compound, various strategies can be applied. These encompass formulations like micro/nanoemulsion suspension or solid dispersions.

Lipidic formulation demonstrating emulsion formation.
At Sygnature Discovery, we possess the expertise, experience, and dedication to drive your molecule towards clinical success. We have the instrumentation onsite to generate everything from lipidic SMEDDS/SNEDDS, spray-dried and lyophilised smorphous solid dispersions, simple solvent–cosolvent systems to nano milling, adding permeation enhancers, PGP inhibitors or combinations thereof to give your material the best chance of success.
The team uses state-of-the-art instrumentation to rapidly test the formulations, such as the Pion µDiss system, which enables simultaneous dissolution and flux measurements.

Pion µDiss system.
Choosing the right formulation for your molecule
With a range of formulation technologies available, choosing the right formulation for your compound is a decision you will need to face. The Form and Formulation team collaborates with customers to select the most suitable formulation solution that ensures the required drug exposure for efficacy and clinical translation. The team will not only advise on the cost of developing a formulation but also the impact on the cost of goods as you proceed to the clinic. Formulations might undergo redevelopment as clients move from Phase I to Phase III. For instance, what works for a Phase I safety study, like a capsule, might not be suitable for a larger Phase III efficacy study where a tablet could be more cost-effective.
Enabling Formulations for Toxicology Studies
The Form and Formulation team can also support customers who require an enabling formulation for toxicology studies. In cases where a customer has chosen a candidate compound with adequate bioavailability to drive efficacy in an in vivo model but experiences non-linear PK, our team can step in.
When a drug exhibits non-linear PK, the increase (or decrease) of dose results in a disproportionate response in free drug concentration at a steady state. For example, doubling the dose won’t lead to a doubling of plasma drug concentration. Non-linear PK can be a factor of absorption, first-pass metabolism, binding, excretion and biotransformation. Where the non-linear PK arises as a factor of absorption or permeation, i.e., an increased dose does not result in a proportional increase in drug plasma concentration, Sygnature Discovery can assist in developing an enabling formulation to enhance the compound’s bioavailability at higher doses.
About the author – David Pearson, Director of the Form and Formulation Department
Dr David Pearson, the Director of Sygnature Discovery’s Form and Formulation Department, is a highly experienced solid form expert with extensive experience at the interface with medicinal chemistry, process chemistry, DMPK and pharmacology. David has spent 20+ years working to support customers to take their molecules from the lab to the clinic. He has achieved this by understanding, controlling and improving the solid-state properties, such as crystallinity, stability and dissolution profiles, of their compounds. David oversees a team at Sygnature whose aim is to deliver a formulation process in a rapid and nimble R&D environment which can then be transferred to cGMP production. David has helped 15+ compounds move to the clinic and beyond.
Meet David at:
Life Science Sweden – New Updates in drug formulation and bioavailability.
Copenhagen 6th September
To arrange a meeting with David in person or remotely, please contact [email protected]
Read more blog articles by David:
Preventing Costly Delays: Accessing Drug Formulation Expertise Early
De-risking Projects with Form Analysis: Pre-clinical Tox Insights
