The Dark Art of Chemistry: Chiral Chromatography
Background of chiral chromatography
Chiral chromatography is a crucial part of the drug discovery process and is used to separate enantiomers. In the 1960s, drugs were marketed as mixtures of enantiomers, an example of this is Thalidomide, a drug used to treat morning sickness in pregnant women. Later, one enantiomer was later discovered responsible for teratogenic effects, leading to severe birth defects in foetuses. While not all cases were as extreme, this example highlights the critical need to separate enantiomers.
In 1974, Professor Barry Karger and his team developed a technique called high-performance liquid chromatography (HPLC) with a chiral stationary phase (CSP) that could separate the enantiomers based on their interactions with the stationary phase. Fast forward ~50 years and within drug discovery this is still being employed albeit with improvements in both column, instrumentation and of course expertise.
Different techniques
There has been and is, lots of interest in chiral molecules. Some are from a purely analytical perspective where techniques such as HPLC, supercritical fluid chromatography (SFC), and capillary electrophoresis (CE) are utilised. In these techniques, the separation is driven by differences in the interaction of the enantiomers with the chiral selector.
Other interests are from a purification perspective. This could be considered a more complex process than analysis due to the need to not only separate but also isolate chirally pure enantiomers. Techniques other than chromatography are also available, including but not limited to crystallisation and enzyme-catalysed reactions. There are also situations where a stereoselective synthesis can be investigated or working from enantiomerically pure starting materials may be more appropriate. Cost vs time is the key criterion on which route to explore.
Within current chromatography groups across the world, speed and quality are of the utmost importance. With the improvements in technology and SFC, chiral screening and purification have become a ‘fast’ and efficient chromatographic process. I should caveat that HPLC still certainly has its place.
Our approach
From 15 years of working with chiral molecules, I believe that optimised screening protocols can only improve the throughput so far. The next step in the improvement of chiral groups will be data review automation. Currently, programmes built ‘in-house’ seem to be the best way forward (maybe a nudge to software vendors here). The implementation of an in-house programme has drastically decreased our analysts’ interaction time (AIT) with the equipment. It is always wise to work smarter, not harder.
Every analyst will have their view on a screening protocol, based on the chemical series they work with and experience. At Sygnature, SFC is the primary chiral platform, and we achieve successful separations on 93% of the samples submitted using the protocol in Fig 1.
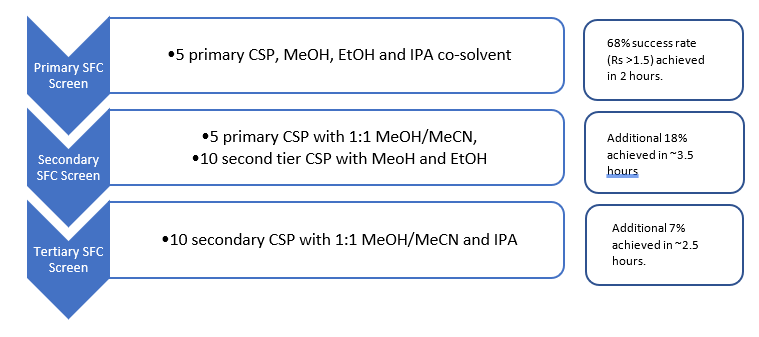
Fig1. Sygnature Discovery SFC chiral screening protocol
One of the critical aspects of chiral screening is identifying the quickest route to the answer without needing multiple systems, which is especially true when receiving upwards of 50 racemates per month. Analysing in parallel is a key component of speed in chiral screening. ‘Chiral pooling’ is the terminology we use for injecting multiple analytes from a single vial and interpreting the SIR channels to identify suitable purification methods. As you can see from Fig 2, separation is observed for compounds with m/z 543 (light blue) and 275 (green), whereas an m/z 554 (brown) is partially resolved (RS<1.5) and m/z 572 shows no separation. m/z 345 does not elute from the column under these conditions. The other masses highlighted are minor achiral impurities.
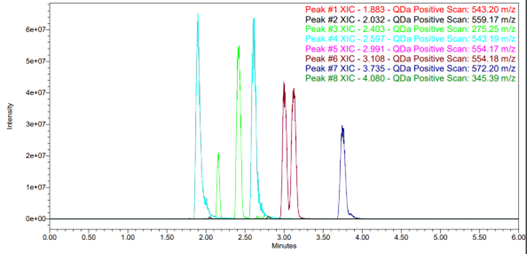
Fig 2. SIR output for 5 pooled chiral racemates using the Chiralpak IC with MeOH
Being able to screen in parallel allows for large time savings. Applying the screening protocol above (60 experiments) takes ~8 hours per compound. From our experience we have not identified any compound-to-compound interactions when pooling, therefore the more analytes screened in parallel, the greater the time saving. Screening of 10 – 20 racemates in an 8-hour period is common practice within our lab.
Purification Workflow
Once a suitable method has been identified from screening, transfer to purification is the next step in the process. Being able to develop multiple methods per day without analyst intervention is a great step in the right direction. However, removing the bottleneck in one part of a process manoeuvres it to another. Multiple parameters can be modified to ‘speed up’ a purification process; loading, buffer concentration, temperature, and BPR pressure, among others. Implementing stacked injections rather than single injections for purification is a key aspect in decreasing turnaround time.
As you can see from Fig 3, the cycle time of a single injection is 11 mins, whereas implementing stacked injections reduces the cycle time to 4.2 mins. This reduces the total purification time by 62%, alongside cost savings in a solvent.
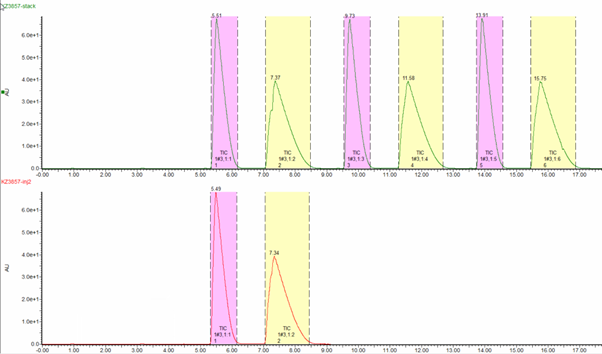
Fig 3. A comparison of single vs stacked injections under SFC conditions. Chiralpak IH 20 x 250mm, 5um. 65g/min, 60/40 CO2 / MeOH (0.2% DEA), 40C, BPR 100 Bar. 42mg per injection.
In conclusion, with the increasing interest in chiral compounds, all automation and time-saving initiatives are critical to the successful support of medicinal chemistry and other industries across the world. Chiral chromatography has the advantage that it is quick compared to other techniques such as asymmetric synthesis or the availability of chirally pure starting materials. The key is to save valuable analysts’ and chemists’ time. Separation Science is crucial in delivering enantiomerically pure material and ensuring the safety and efficacy of drugs and agrochemicals which is a critical regulatory requirement.
About the author
Alex Brien is a Senior Principal Scientist in Analytical Chemistry with 15 years of experience in chiral chromatography.
