The Rule of 5 – Two decades later
It’s now over 20 years since Pfizer’s Chris Lipinski introduced the Rule of 5 to describe drug-like molecules. Rather than a set of hard and fast rules, it provides a guideline that points designers towards molecules whose properties make them more likely to succeed in the clinic. Sygnature Discovery’s Dr Steve St Gallay shares his thoughts on the ‘rule’.
The concept is simple. To be drug-like, a molecule should have no more than five hydrogen bond donors, no more than 10 hydrogen bond acceptors, a molecular weight less than 500 Da, and log P below 5 to ensure it is not too lipophilic. Slavish compliance is not essential. Indeed, this may lead to otherwise promising lead molecules being discarded unnecessarily. But the Rule has made people much more aware of the properties that may lead to success. 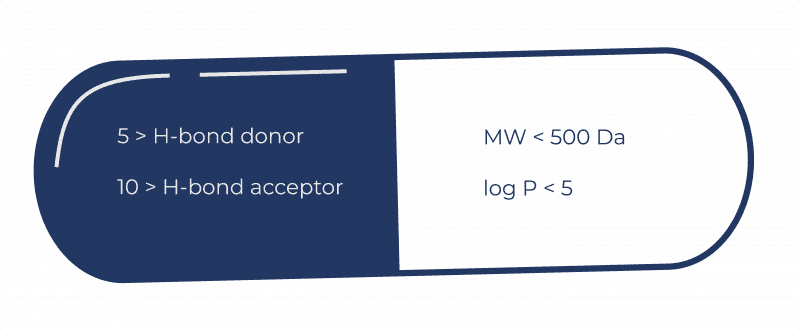
For each project, what parameters will give a molecule the properties we require? As well as docking with its target, a drug has to be efficacious, safe, and able to reach the target site in the patient. Drug design is a multi-parameter optimisation process. Greater lipophilicity may well increase potency, but once it heads above 5, the probability that it will be a successful drug starts to reduce – its solubility will fall, for example, and it’s more likely to be metabolised by liver enzymes.
Exploring new areas of chemical space can take us outside Lipinski’s guidance, but it is important to remember why the Rule is there. A molecule that complies will almost certainly have many other drug-like properties: successful drugs are typically smaller and less lipophilic. If we need to go outside the Rule of 5 landscape, there should be a good reason for it. For example, we might need to make a slightly larger molecule to achieve the desired potency, but this might make our molecule less soluble or less permeable.
The Rule of 5 reminds scientists to think carefully about the properties they are designing into their molecules. With all else being equal, given one molecule with a molecular weight of 450 and another that comes in at 550, you’d likely go for the smaller one. But if that 550 is truly necessary for efficacy, it may well still make it to the market.
Increasing numbers of compounds that go beyond the Rule of 5 are now being developed and reaching the market. The new generations of molecules such as macrocycles and ProTacs, for example, are a step beyond what Lipinski was considering 20 years ago. Science progresses!
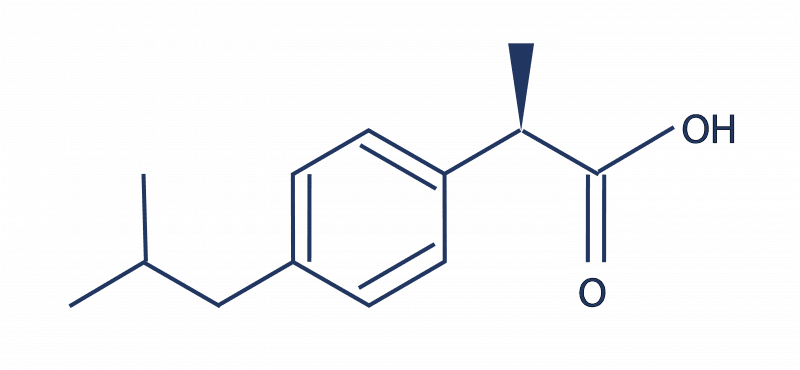 Sygnature has one of the largest medicinal chemistry groups in the UK, and is hosting an event to celebrate 20 years of the Rule of 5 on 20th November 2019. The event, with an international panel of expert speakers, is taking place at our head-quarters in Nottingham. It’s doubly appropriate, as this site is where one of the most successful drugs in history, ibuprofen, was invented, by chemists at Boots. With a molecular weight of 206, just two hydrogen bond donors and one acceptor, and a log P of just under 4, it is fully Rule of 5 compliant.
Sygnature has one of the largest medicinal chemistry groups in the UK, and is hosting an event to celebrate 20 years of the Rule of 5 on 20th November 2019. The event, with an international panel of expert speakers, is taking place at our head-quarters in Nottingham. It’s doubly appropriate, as this site is where one of the most successful drugs in history, ibuprofen, was invented, by chemists at Boots. With a molecular weight of 206, just two hydrogen bond donors and one acceptor, and a log P of just under 4, it is fully Rule of 5 compliant.
As always, if you would like to speak to us about this topic or anything and everything drug discovery, we’d love to hear from you. Feel free to get in touch using our contact form.
