Corcept Therapeutics: Integrated Drug Discovery Collaboration
Corcept Therapeutics, located in Menlo Park, CA, is a leader in the discovery and development of drugs that regulate the effects of cortisol. Corcept’s scientific expertise surrounding the regulation of cortisol has led to the discovery of an extensive library of novel compounds that may ultimately address life-threatening diseases such as Cushing’s syndrome, caused by excessive activity of cortisol, and cancer, including prostate and pancreatic cancers.
Collaboration
Sygnature’s long-term integrated drug discovery collaboration with Corcept began at the lead optimisation phase. Our pharmaceutical industry research-experienced scientists delivered innovative medicinal chemistry alongside a comprehensive, diverse screening cascade of in vitro assays, including potency, selectivity, mode-of-action and ex vivo biomarker.
Lead compounds were further profiled in collaboration with our expert alliance partners, Saretius and Renasci, to obtain pivotal in vivo PK and PD data prior to advancing drug candidates into man.
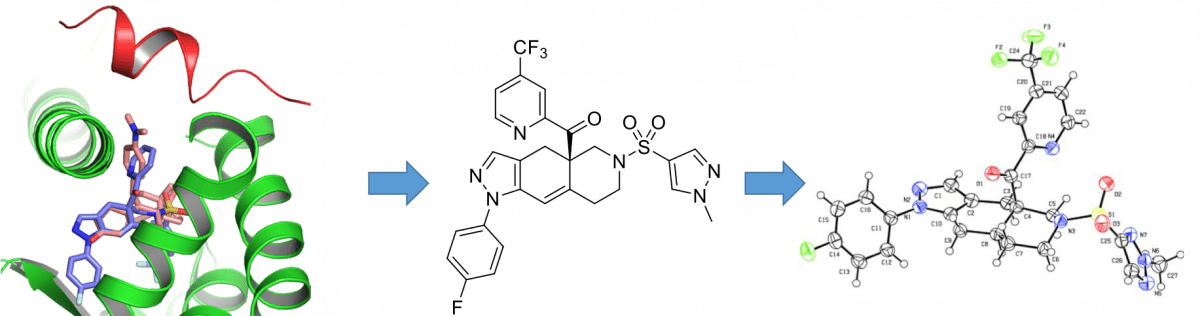
Deliverables
- Sygnature has, so far, delivered two clinical development candidates to Corcept. Both compounds are potent, selective glucocorticoid receptor antagonists designed for oral administration:
- Relacorilant (previously known as CORT125134) – Phase II study in patients with Cushing’s syndrome and a Phase I/II clinical trial in combination with Abraxane® in patients with solid tumours, including pancreatic cancer
- CORT125281 – Phase I trial in healthy subjects and a dose-ranging clinical study in combination with Xtandi® (enzalutamide) in patients with castration-resistant prostate cancer
- Sygnature has also developed a novel patented biomarker assay of cortisol activation (WO 2016187347). Methods are provided for assessing a clinical response to a glucocorticoid receptor antagonist (GRA) in a human subject and for diagnosing Cushing’s syndrome
- Sygnature’s former Director of Chemistry, Dr Iain Walters, presented the discovery and first disclosure of CORT125134 at the prestigious 19th Royal Society of Chemistry / Society of Chemical Industry Medicinal Chemistry Symposium (10th-13th September 2017, Cambridge, UK) – http://www.rsc.org/events/detail/23597/19th-rsc-sci-medicinal-chemistry-symposium )
Publications and Patents – Corcept and Sygnature
Hunt, Hazel J.; Belanoff, Joseph K.; Walters, Iain; Gourdet, Benoit; Thomas, Jennifer; Barton, Naomi; Unitt, John; Phillips, Timothy; Swift, Denise; Eaton, Emily. The Identification of the Clinical Candidate (R)-(1-(4-fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1Hpyrazolo[3,4-g]isoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methanone (CORT125134):– A Selective Glucocorticoid Receptor (GR) Antagonist. Journal of Medicinal Chemistry (2017), 60(8), 3405-3421
Bali, U., Phillips, T., Hunt, H., and Unitt, J. FKBP5 mRNA Expression Is a Biomarker for GR Antagonism. Journal of Clinical Endocrinology & Metabolism (2016), 101(11), 4305-4312017), 60(8), 3405-3421.
Belanoff, Joseph K.; Hunt, Hazel; Unitt, John Francis; Moraitis, Andreas G. From PCT Int. Appl. (2016), WO 2016187347 A1 20161124.
Hunt, Hazel J.; Belanoff, Joseph K.; Golding, Emily; Gourdet, Benoit; Phillips, Timothy; Swift, Denise; Thomas, Jennifer; Unitt, John F.; Walters, Iain. 1H-Pyrazolo[3,4-g]hexahydro-isoquinolines as potent GR antagonists with reduced hERG inhibition and an improved pharmacokinetic profile. Bioorganic & Medicinal Chemistry Letters (2015), 25(24), 5720-5725.
Hunt, Hazel; Walters, Iain; Ray, Nicholas. From PCT Int. Appl. (2015), WO 2015077537 A1 20150528.
Hunt, Hazel; Walters, Iain; Gourdet, Benoit. From PCT Int. Appl. (2015), WO 2015077530
Hunt, Hazel; Johnson, Tony; Ray, Nicholas; Walters, Iain. From PCT Int. Appl. (2013), WO 2013177559
