Dietary-Induced obese (DIO) mice and rat model
Our DIO mouse model is ready to use, enabling a quick start to your study and a seamless transition from the DIO induction phase to the testing of novel treatments.
Key features of our DIO mouse model are:
- Continuously available (minimal lead in time)
- Weight stable (allowing assessment of weight loss rather than inhibition of weight gain)
- Marked adiposity (30-40% fat)
- Moderate insulin resistance
- Ectopic lipid deposition and liver steatosis
- Elevated plasma lipids
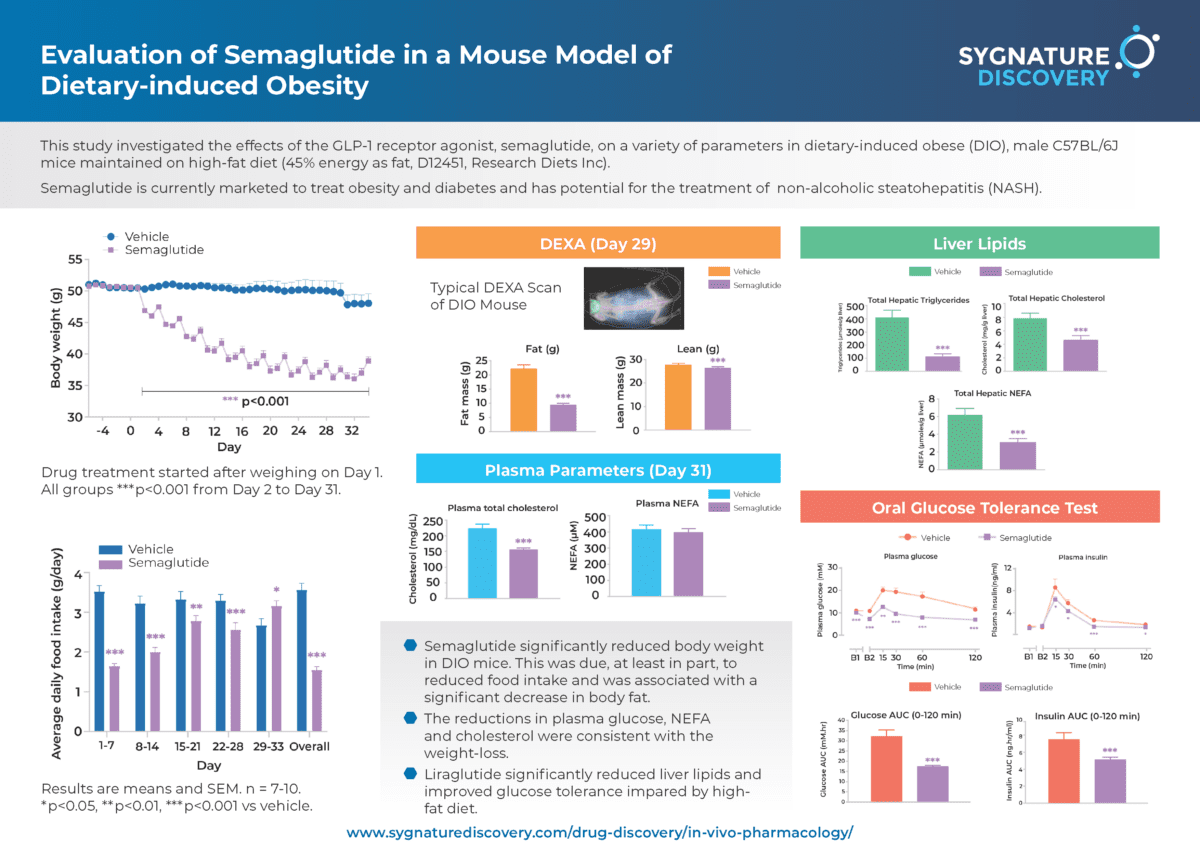
Below is an example of a typical 4-week study using the DIO mouse model, which demonstrates the efficacy of the clinically effective GLP-1 receptor agonist, semaglutide.
We have also developed and validated a DIO rat model using female Wistar rats allowed to develop obesity by ad libitum access to a simplified cafeteria diet, consisting of high-fat chow, ground chocolate and ground peanuts to mirror a typical calorie-dense Western diet. The DIO rat model has excellent predictive validity and is produced on demand, with a 16-week lead time.
Twice-weekly administration of semaglutide reduces body weight in the dietary-induced obese (DIO) mouse
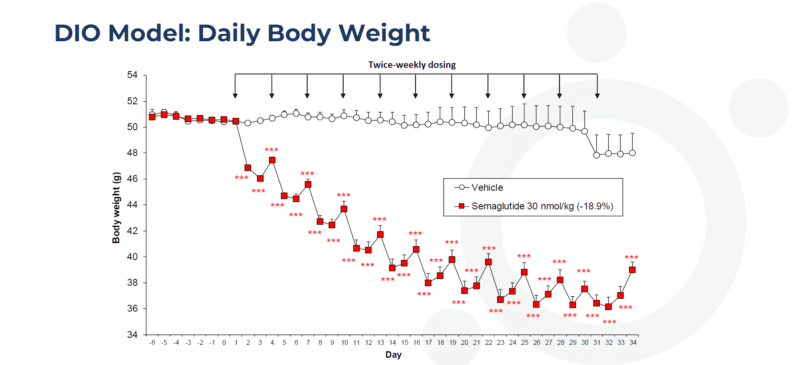
Data are adjusted means (n=10). SEMS are calculated from the residuals of the statistical model. Data analysed by ANCOVA with body weight on Day 1 as covariate. Comparisons to vehicle were by multiple t test (***p<0.001). Values in parentheses are % change from vehicle on day 34. Arrows indicate dosing days.
Twice-weekly administration of semaglutide reduces cumulative daily food intake in the dietary-induced obese (DIO) mouse
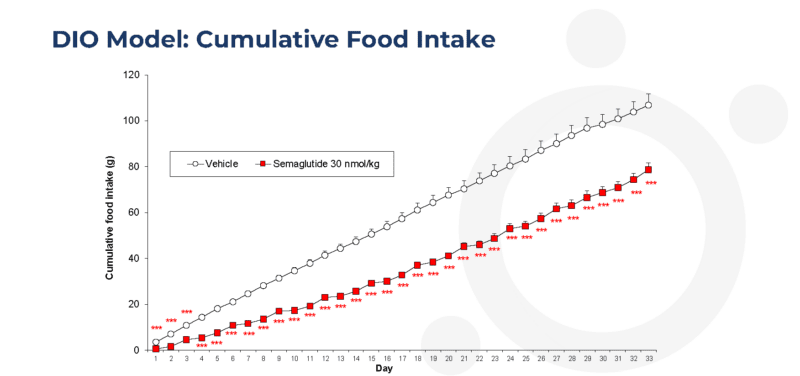
Data are adjusted means (n=10). SEMs are calculated from the residuals of the statistical model. Data analysed by ANCOVA with average food intake from Day -6 to Day p as covariate. Comparisons to vehicle were by multiple t test for (***p<0.001).
Twice-weekly administration of semaglutide reduces total adiposity in the dietary-induced obese (DIO) mouse
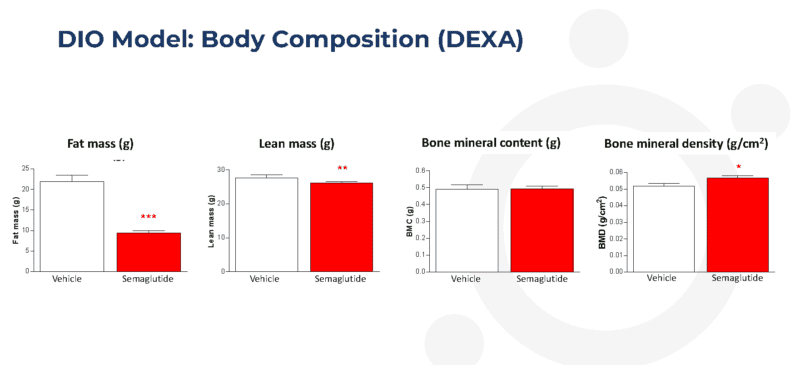
Body composition was performed by DEXA on mice anaesthetized with isoflurane using the Lunar PIXImus Densitometer on Day 28. Statistical analysis was by robust regression with treatment as a factor and Day 1 body weight and scanning order as covariates. Data are shown as adjusted means (n=10) and standard errors of the mean (SEM) were calculated from the residuals of the statistical models. Comparisons of treatments to the vehicle were by the multiple t-test for Semaglutide. Significant differences against the vehicle are denoted by *p<0.05,**P<0.01, ***P<0.001.
Twice-weekly administration of semaglutide reduces liver lipid content in the dietary-induced obese (DIO) mouse
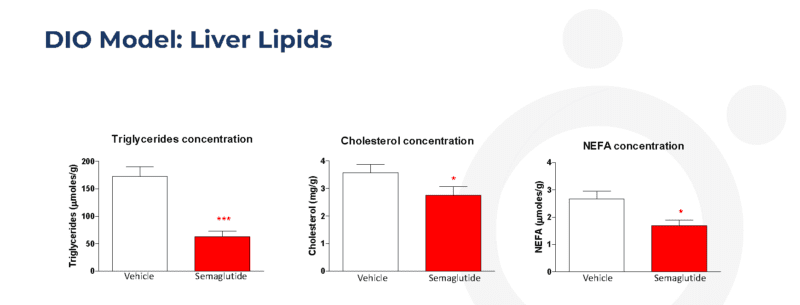
Data are shown as adjusted means (n=10) and SEM. Analysis was by robust regression or general linear model with treatment and termination team as a factor and Day 1 body weight and termination order as covariates. Comparisons of to vehicle were by the multiple t-test for Semaglutide. *p<0.05 and ***p<0.001.
Twice-weekly administration of semaglutide improves glucose tolerance in the dietary-induced obese (DIO) mouse
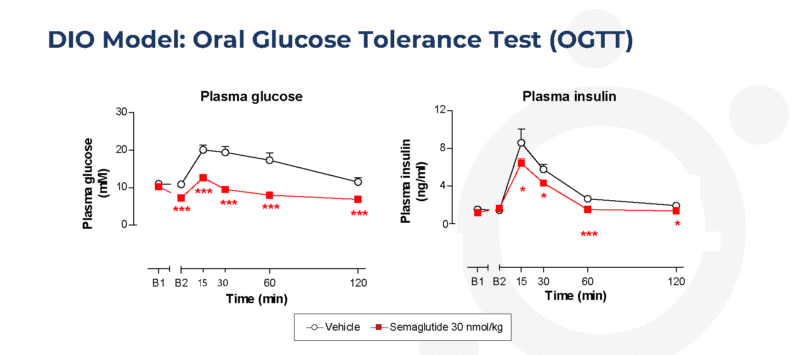
Data are shown as adjusted means (n=10) and standard errors of the mean (SEM) are calculated from the residuals of the statistical models. Analysis was by robust regression of log-transformed data with treatment as a factor, and Day 1 body weight and termination order as covariates. Comparisons of the vehicle were by the multiple t-test for Semaglutide *p<0.05, **p<0.01 and ***p<0.001.
Twice-weekly administration of semaglutide improves the plasma cholesterol profile in the dietary-induced obese (DIO) mouse
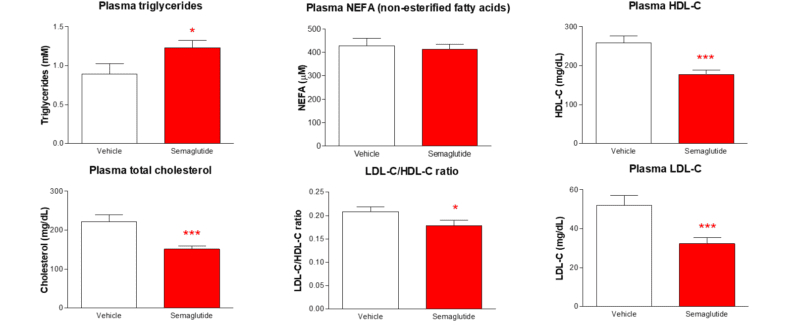
Data are adjusted means (10) and standard errors of the mean (SEM) are calculated from the residuals of the statistical models. Analysis was by robust regression or general linear model of untransformed data with treatment as a factor and bleeding order and day 1 body weight as covariates. Comparisons to vehicle were by the multiple t-test for Semaglutide *p<0.05 ***p<0.001.
Studies in dietary-induced obese rats and mice are customised for each client and can include measurements of various parameters.
Get in touch for more information or browse our models of metabolic disorders.