LogD (Micro Shake-Flask)
Highly lipophilic compounds (logD7.4 > 3.5) are likely to have:
- Poor aqueous solubility that can compromise intestinal absorption
- High first-pass metabolism, leading to high in vivo clearance and contributing to low oral bioavailability
- High binding to plasma proteins or tissues
- Increased potential to inhibit Cytochrome P450 enzymes, especially CYP3A4, increasing the risk of drug-drug interactions (DDIs)
- Interactions with transporters e.g. P-glycoprotein, again increasing the risk of drug-drug interactions
- Significant binding to glass and plastic, compromising in vitro experiments
On the other hand, within a series of compounds lipophilicity often shows a positive correlation with permeability and potentially oral absorption. Similar correlations are often observed between logD7.4 and potency at the pharmacological target.
Designing new compounds with the right balance of lipophilicity is therefore paramount to get the right ADME profile.
Experimentally, a compound’s lipophilicity is measured by its distribution between an aqueous phase (usually phosphate buffer at pH 7.4) and a water-immiscible organic solvent (usually 1-octanol). After a period of vigorous mixing, phase separation is achieved by centrifugation and the compound concentrations in both solvents are measured by LC-MS/MS.
LogD7.4 is then calculated using the following equation:
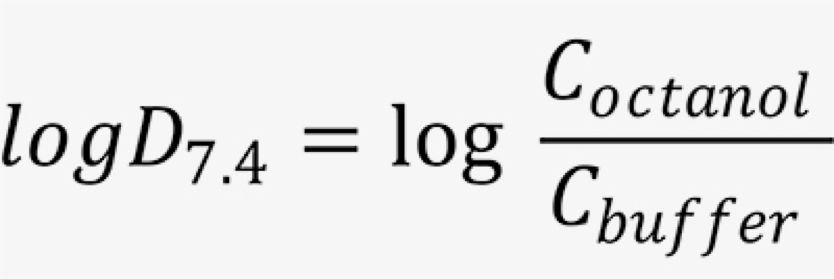
Where Coctanol is the solute concentration in octanol and Cbuffer is the solute concentration in buffer.
It is very important to keep in mind that for compounds with an ionisable group, i.e. acids and bases, logD7.4 is strictly dependent on the ionisation state and, therefore on the pH value of the aqueous buffer.
