Side Effect Profiling
We offer a wide range of behavioural and physiological assays that can be used to assess the mode of action, efficacy and side-effect profile of centrally acting drugs. They can also be used in proof-of-concept studies for novel molecular targets in the brain. These assays include:
- Behavioural phenotyping (transgenic animals)
- Initial safety assessment of novel compounds
- Irwin test
- Rotarod
- Neurotransmitter-specific functional tests
- Measurement of the effects of drugs on seizure threshold
- Pupillometry
Drugs (alone or in combination with a receptor agonist or antagonist) can be given by a variety of different routes (including intracerebroventricular injection). Blood and tissue samples can be collected for pharmacokinetic or neurochemical analysis or ex vivo binding if required.
Rotarod for assessment of motor function
Diazepam reduces fall latency in the rat rotarod test
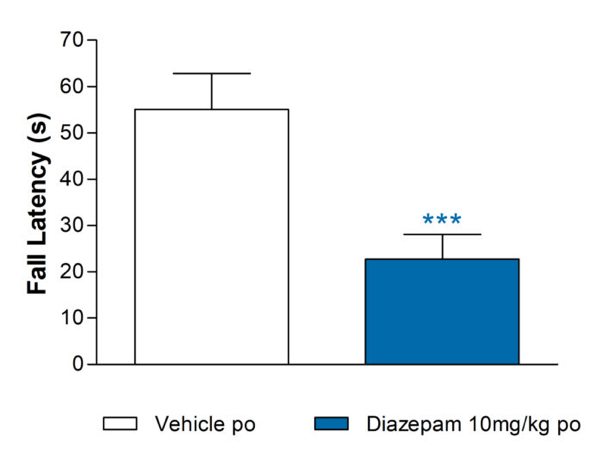
Data are back-transformed adjusted means (n=12). SEMs are calculated from the residuals of the statistical model.
Data was analysed by ANOVA on square root transformed data averaged over trials 1-3 for each animal.
Comparisons to vehicle for Diazepam by Williams’ test ***p<0.001.
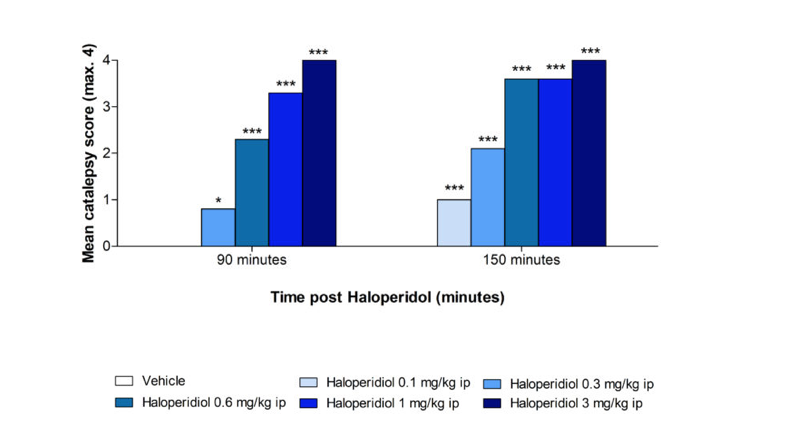
Catalepsy assessment for evaluating extrapyramidal side effects
The typical antipsychotic Haloperidol induces catalepsy in rats
Pupillometry for assessment central function
Adding to our portfolio of CNS services, we have recently developed a pupillometry model of morphine- and clonidine-induced mydriasis. This assay can be utilised to better understand the pharmacology of novel centrally acting drugs, and as a measure of parasympathetic and sympathetic tone.
Clonidine-induced mydriasis in rats
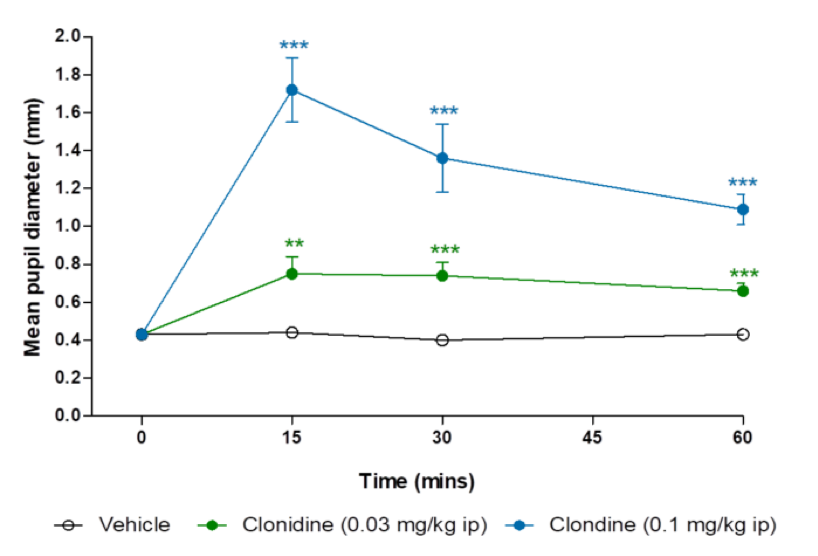
Data are back-transformed adjusted means ± SEM (n=8). Analysis was by two-way analysis of covariance
of log transformed data with treatment and day as factors and pupil diameter at 0 mins as a covariate.
Comparisons against vehicle were by Williams’ test, **p<0.01, ***p<0.001.
Morphine-induced mydriasis in rats
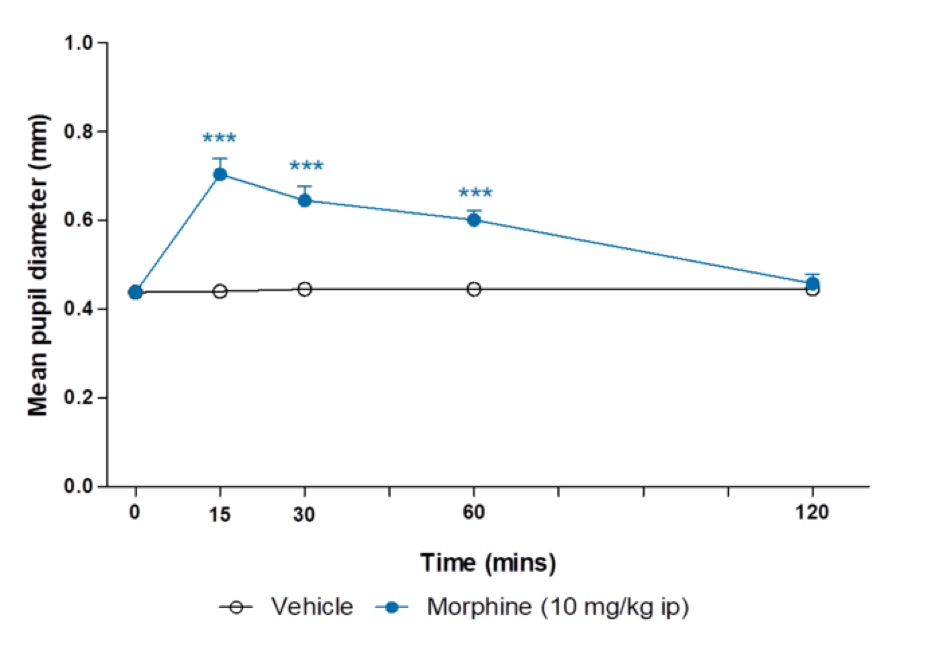
Data are back-transformed adjusted means ± SEM (n=8-16). Analysis was by one-way analysis of covariance
of log transformed data with treatment as a factor and pupil diameter at 0 mins as a covariate.
Comparisons against vehicle were by multiple t-test ***p<0.001.
Measurement of Prolactin
Increased plasma prolactin can be a side-effect of antipsychotic drug administration, typically associated with Dopamine D2 receptor antagonism. We offer a plasma prolactin assay that can assess whether novel drugs to treat schizophrenia increase circulating levels of this pituitary hormone.
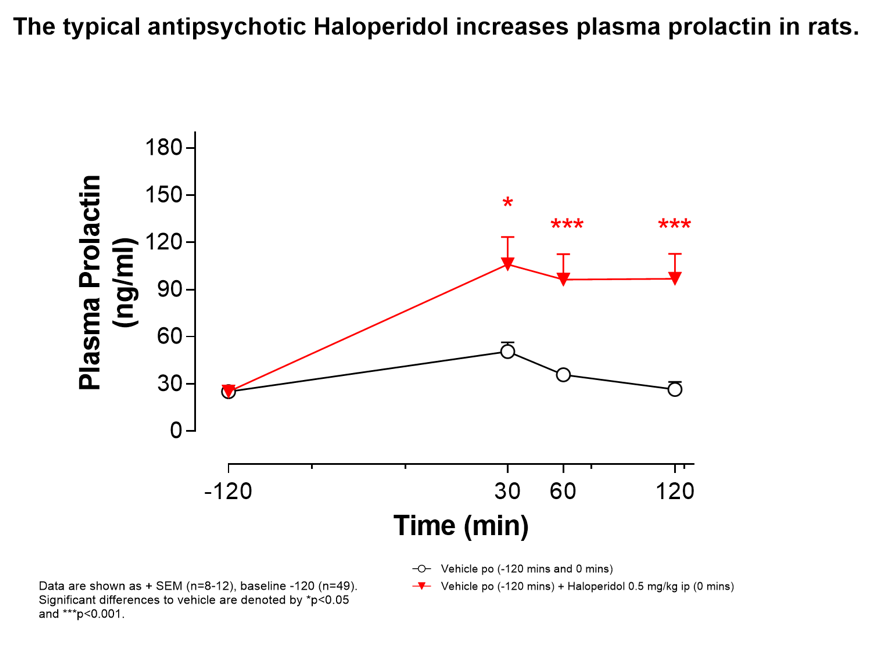
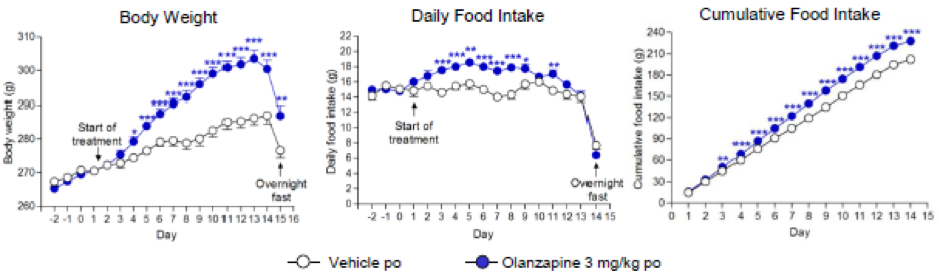
Weight Gain
Weight gain can be a serious side-effect of antipsychotic treatment. We have developed a rat model of antipsychotic weight gain.
Effect of olanzapine on various parameters in female rats maintained on high fat diet